|
|
|
pKalc
Customer Benefits
This program provides an indispensable resource for predicting acidic and basic pKa values before synthesis, such as in diversity calculations for combinatorial chemistry, in QSAR, in evaluating the synthesis possibilities, or in characterizing substance libraries. It is also helpful in situations when compounds are poorly soluble or may decompose in a water solution, as well as in the case of pKa values which are too high or too low.
Brief Description
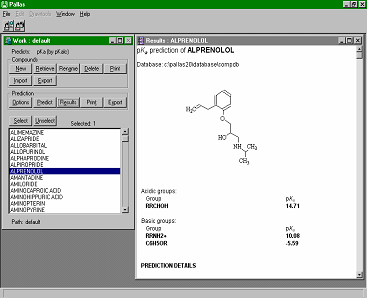 CompuDrug's pKalc calculates the accurate acidic and basic pKa values (negative logarithms of acid-base ionization constants) for organic compounds, in most cases, within an error of 0.25 pKa units. The calculation can be performed for any organic compound, including aromatics, mono and polyheteroaromatics, and small peptides. The applied logarithm, adapted after Hammett and Taft takes into account all necessary electronic, steric and other effects and relies on an extended database of almost a thousand equations. CompuDrug's pKalc calculates the accurate acidic and basic pKa values (negative logarithms of acid-base ionization constants) for organic compounds, in most cases, within an error of 0.25 pKa units. The calculation can be performed for any organic compound, including aromatics, mono and polyheteroaromatics, and small peptides. The applied logarithm, adapted after Hammett and Taft takes into account all necessary electronic, steric and other effects and relies on an extended database of almost a thousand equations.
Features at a Glance
-
Predicts pKa values in aqueous phase
-
Indicates acidic and basic pKa values
-
Highlights the substructures corresponding to each pKa value.
-
Implemented Wizard for develping new Hammett-Taft equations.
-
Uses large KB (more than 600 ionizable centres and 800 substituents)
-
Lowest acidic and highest basic pKa values are considered
-
Effect of ionized substituents are taken into account
-
Exporting results to any spreadsheet editor (in TAB delimited text file format)
pKalc upgrades
-
In order to improve the accuracy of pKa predictions in pKalc software, the majority of acidic and basic groups have been re-trained, using more than 10,000 experimental pKa values collected from the chemical literature.
Thanks to the new inserted parameters, the upgraded version of pKalc provides significantly more accurate predictions.
The upgrade appears in Pallas for Windows, Pallas for Linux/Unix, Pallas Net, ISIS Plug-in, and Pallas SDK applications.
-
Acid-base properties and protonation-deprotonation equilibria are among the best known chemical phenomena. Observations of the effects of changing the molecular structure on acid-base equilibria have provided much of the theoretical foundation of modern organic chemistry. Many chemists, like analytical chemists, organic chemists, physical chemists, biochemists or molecular biologists often need to know the acidity or basicity of organic compounds, (i.e. the pKa values of the molecules). Sometimes difficulties may arise in the experimental determination of pKa values because it is time-consuming and requires laboratory experience.
In order to improve the accuracy of pKa predictions of the pKalc software, the further acidic and basic groups have been re-parameterized using more than 5,000 experimental aqueous pKa values collected from the chemical literature. The upgrade has significantly increased the accuracy of the prediction, and covers a wider range of the chemical space.
pKalc is operating under the following computing platforms: Windows/Linux, Web application, Software Development Kit
If you need more information about our product, please e-mail CompuDrug International at abc@compudrug.com

|



 CompuDrug's pKalc calculates the accurate acidic and basic pKa values (negative logarithms of acid-base ionization constants) for organic compounds, in most cases, within an error of 0.25 pKa units. The calculation can be performed for any organic compound, including aromatics, mono and polyheteroaromatics, and small peptides. The applied logarithm, adapted after Hammett and Taft takes into account all necessary electronic, steric and other effects and relies on an extended database of almost a thousand equations.
CompuDrug's pKalc calculates the accurate acidic and basic pKa values (negative logarithms of acid-base ionization constants) for organic compounds, in most cases, within an error of 0.25 pKa units. The calculation can be performed for any organic compound, including aromatics, mono and polyheteroaromatics, and small peptides. The applied logarithm, adapted after Hammett and Taft takes into account all necessary electronic, steric and other effects and relies on an extended database of almost a thousand equations.
